Hypoglycaemic, Insulin Modulation and Antioxidant Potentials of Ricinus communis L. Extracts in Streptozotocin-Induced Diabetes Male Wistar Rats
| Received 21 May, 2024 |
Accepted 11 Jun, 2024 |
Published 12 Jun, 2024 |
Background and Objective: Diabetes is a multifaceted disorder causing insulin resistance, hyperglycemia and impaired glucose tolerance leading to complications such as oxidative stress. This study evaluates the therapeutic effects of methanolic leaf extract of Ricinus communis L. (MERC) on streptozotocin (STZ)-induced diabetic male Wistar rats, comparing its efficacy with metformin. Materials and Methods: A total of 35 rats were split up into 5 groups: G1 rats were given dimethyl sulphuroxide (DMSO); G2 rats were given STZ-induced diabetes; G3 rats were given metformin; G4 rats were given MERC treatment; and G5 rats were given MERC alone for 21 days. Following the experiment, blood samples were taken for measurements of blood glucose, insulin and glycated haemoglobin (HbA1c). Oxidative stress biomarkers such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and lipid peroxidation’s index malonaldehyde (MDA) were assayed. Results: Along with decreased antioxidant enzyme levels, G2 demonstrated significant increases (p<0.05) in FBG, HbA1c, oxidative stress markers. Compared to G2, G5 displayed improvements in oxidative stress markers and insulin levels. Comparing G4 to G3, the latter showed higher (p<0.05) levels of antioxidant enzymes and decreased levels of FBG.Conclusion:Methanolic extract of Ricinus communis L. significantly improved blood glucose and antioxidant status in diabetic male Wistar rats, comparable to metformin and sometimes even better than metformin. This suggests potential as a natural alternative or complementary diabetes treatment, warranting further research and clinical trials in humans.
| Copyright © 2024 Dimeji et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Diabetes is a serious health problem all over the world. It causes high levels of sugar in the blood and can lead to illness and death. The chronic metabolic disease known as type 2 diabetes mellitus (DM) has been becoming more common worldwide. Because of this trend, it is swiftly expanding to other regions of the world and, as a result of an ageing population, is expected to afflict twice as many individuals in the next ten years. This will increase the burden already placed on healthcare providers, particularly in less developed nations1. The multifactorial nature of diabetes involves complex interactions between genetic, environmental and lifestyle factors. Nigeria has the highest percentage of DM in Africa2. Estimates for 2020 put the prevalence of diabetes and its complications in Nigeria at 3%, but some studies argue that the International Diabetes Federation (IDF) underestimated the severity due to extrapolated data3. Hospital reports show that 223 out of 100,000 people in Nigeria are treated annually for diabetes-related complications, with 22% of these patients dying4. Prenatal impacts, infections, genetic abnormalities, certain drugs, insulin resistance (T2DM) and insulin insufficiency (T1DM) are some of the possible causes5. Suh et al.6 indicate that chronic diabetes impairs organ function or causes organ failure, contributing significantly to severe complications such as diabetic renotoxicity.
The streptozotocin (STZ), a naturally occurring alkylating anticancer drug, contains glucosamine-nitrosourea and is particularly toxic to pancreatic β-cells in mammals by causing DNA damage. In addition to DNA damage, STZ activates poly (ADP-ribose) polymerase (PARP) due to DNA degradation7. The increased amount of GLUT2 in the cell8 makes β-cells poisonous as a result. STZ is used to treat metastatic pancreatic islet cell cancer and in studies using animal models of hyperglycemia9. The FDA has approved STZ for use, but due to its toxicity and rare cancer cure potential, it is only used for non-surgically curable cancer patients. Tumor sizes and malignant symptoms, particularly hypoglycemia, have significantly decreased with STZ use10.
Various animal species are susceptible to STZ’s pancreatic β-cell cytotoxic effects, with rabbits being less affected than mice, rats and monkeys. STZ is more effective in inducing diabetes in rats and mice11. In diabetes, hyperglycemia speeds up the generation of reactive oxygen species (ROS), which causes oxidative stress and consequent cellular damage12. Lipid peroxidation, protein degradation and DNA oxidation are the results of this oxidative stress, which upsets the equilibrium between pro-oxidant and antioxidant systems13. Retinal problems in diabetes must be prevented or mitigated with effective oxidative stress management. Type 2 diabetes is treated with the biguanide metformin initially. It exhibits protective properties against diabetic renotoxicity by increasing insulin sensitivity and lowering hepatic glucose production14. Metformin does not, however, come without adverse effects. In rare instances, prolonged usage of the medication can result in lactic acidosis and gastrointestinal problems15. Finding complementary or alternative therapy is therefore essential to improving the management of diabetes.
The potential of medicinal plants to treat diabetes and its consequences has been investigated. Since ancient times, herbal plants have been used for healing, especially in emerging and impoverished nations where synthetic medications are less accessible16. Approximately 65-70% of India’s rural population relies on medicinal plants for healthcare. The use of herbal supplements and pharmaceutical products has increased dramatically over the last three decades, to the point where at least 80% of people worldwide now depend on them for some form of basic healthcare16. Numerous herbal medicines have been proven to be effective and treatments including these substances have shown a great deal of promise. However, many of them are still unproven and their usage is either inadequate or not monitored at all. Herbal mixtures are preferred for their effectiveness and fewer side effects compared to synthetic medications17.
Ricinus communis L. or the castor plant, is part of the Euphorbiaceae family and is used worldwide for medicinal purposes18. Industrial applications for castor oil include lubrication, preservative and inclusion in paints, varnishes, waxes and polishes. Castor oil is now permitted by the FDA to be used as a flavouring and food additive19. Numerous phytochemicals are present in Ricinus communis L.19,20 and these phytochemicals are responsible for the plant’s pharmacological and therapeutic qualities, which include
anticancer, antimicrobial, insecticidal, antioxidant, anti-diabetic, anti-nociceptive, anti-inflammatory, bone-regenerative, analgesic and anticonvulsant effects21. Numerous molecular pathways have been demonstrated to be targeted by these phytochemicals22. Due to its rich phytochemical composition, pharmacological activity and supporting clinical trials, Ricinus communis L. holds potential as a source for the development of medicinal pharmaceuticals18.
Diabetes mellitus is a long-term metabolic disease characterized by disturbances in insulin action, secretion, or both, leading to persistent hyperglycemia. Diabetes is becoming increasingly common worldwide, making it a serious public health problem. Although metformin and other current pharmaceutical therapies are successful, they also have disadvantages such as high prices, drug resistance and side effects. Therefore, research is ongoing to find complementary or alternative medicines that are cheaper, easier to use and have fewer negative effects. Castor or Ricinus communis L. is a plant known for its therapeutic properties. Many civilizations have long used castor seed for its analgesic, antibacterial and anti-inflammatory properties21. Recent research has shown that many parts of the castor bean plant may have blood sugar-lowering effects, suggesting that it may be useful in treating diabetes. Plant extracts such as those from Ricinus communis have been shown in the past to contain bioactive substances such as flavonoids, alkaloids and glycosides20, which may have anti-hyperglycemic properties. According to this research, methanolic castor extract can act as an antioxidant, regulate blood sugar levels and increase insulin sensitivity, all of which help protect pancreatic β-cells from damage caused by oxidative stress. For type 2 diabetes, metformin is a recognized first-line drug that effectively lowers blood sugar levels and increases insulin sensitivity. However, comparing the therapeutic benefits of Ricinus communis L. methanolic extract with metformin may shed light on the plant’s potential as a replacement or adjunctive treatment. A study like this would compare and contrast the benefits of the natural extract and see if it may provide the same or better results with fewer side effects.
It is hypothesized that the methanolic extract of Ricinus communis L. (castor bean) will exhibit significant antihyperglycemic and antioxidant effects comparable to or greater than those observed with metformin treatment in streptozotocin-induced diabetic male Wistar rats. The study objectives include to access the impact of Ricinus communis L. extracts on blood glucose level and Insulin concentration in streptozotocin-induced diabetes male Wistar rats and to determine the impact of Ricinus communis L. extracts on oxidative stress biomarkers such as SOD, CAT, GPX and lipid peroxidation’s index MDA in streptozotocin-induced diabetes male Wistar rats.
This study also compares the efficacy of Ricinus communis L. extracts with the standard anti-diabetic drug, metformin in the treatment and management of diabetes complications.
MATERIALS AND METHODS
Study duration: This study was conducted between March and May, 2023.
Experimental animals: A total of 35 male Wistar rats, weighing between 200 and 250 g, were acquired from the Department of Physiology’s animal house at LAUTCH, Ogbomoso and housed there for two weeks while they adapted. Because there were no signs of stress or infection, these rats were deemed healthy because they had not received any previous experimental treatments. The rats were individually weighed before to the start of the trials following a two-week acclimatization phase. They were kept in the animal facility of the Department of Physiology, Faculty of Basic Medical Sciences, LAUTECH, Ogbomoso, Oyo State, in well-ventilated plastic cages. The animals in the study were fed a regular food and given water, with a 12-hrs light/dark cycle, a temperature range of 25±20°C and a humidity level of 60-65%. The guidelines provided in the National Research Council’s Guide for the Care and Use of Laboratory Animals were followed throughout the experimental protocols.
Ethical clearance: Ethical clearance was sought and given by the Faculty of Basic Medical Sciences (FBMS) Ethical Committee, Ladoke Akintola University of Technology, Ogbomoso with reference number ERCFBMSLAUTECH: 016/12/2023.
Chemicals and drugs
Streptozotocin: Streptozotocin labeled Ottokemi Biochemika S 2600 Streptozotocin, 98% C8H15N3O7, MW 265.22, 188883-66-4 powder was purchased from Mr. David, Aroje area, Ogbomoso.
Dimethyl sulfoxide (DMSO): Thermo Fisher Scientific manufactures this organosulfur compound in India under the formula (CH3)2SO. Trade in Masifa, Ogbomoso and DMSO was obtained. Dimethyl sulfoxide (DMSO) is a frequently used drug delivery vehicle because of its miscibility in a wide range of organic solvents, including water and its capacity to interact with both polar and nonpolar molecules. Because it serves as a vehicle for the delivery of a certain chemical, DMSO is used as a control. This was done in an attempt to determine if the DMSO or the chemical under test was responsible for any alterations seen23.
Metformin: Metformin hydrochloride (DIAMIN), which is manufactured by Cris Pharma (India) Limited, was acquired from Ogbomoso’s Akol pharmacy. In 1% distilled water per animal’s body weight, it was dissolved.
Plant collection and identification: Ricinus communis leaves were harvested from a farmland at Iluju, Ogbomoso, Oyo State, Nigeria. This plant was identified by Mrs. Ogundola a Botanist in the Department of Pure and Applied Biology LAUTECH, Ogbomoso bearing voucher number LHO677 and the specimen was deposited at the herbarium of the institution.
Extract preparation: The leaves were meticulously washed twice with running water and once with distilled water, Following a week of air drying (out of direct sunshine), it was dried in an oven at a temperature below 500°C and ground with an electric mill to a fine powder. It was kept in an airtight container so that additional processing would be easier. The 700 g of powdered plant material was put into a 5L container with 96.6 % methanol. After that, the container was securely shut and let to settle for 48 hrs. The mixture was sieved through muslin after 48 hrs. The tar was discarded and the mixture was passed through filter paper for further extraction. The obtained filtrate was passed through a rotary evaporator to concentrate the extract after methanol boiled at 64.7°C. The extract was then air-dried and reconstituted in DMSO solution prepared at a ratio of 1:19 water for oral administration via an oral cannula24.
Diabetes induction: The animals in groups 2, 3, and 4 were induced by a single intraperitoneal injection of streptozotocin (STZ, 55 mg/kg, freshly generated in 0.1 M citrate buffer pH 4.5) following acclimatization prior to extract administration. Streptozotocin should induce diabetes in three days by destroying the beta cells. Group 1 received an equivalent volume of vehicle 0.1 M DMSO, pH 4.5. Using an accu-chek glucometer, the animals’ FBG was assessed 72 hrs later to confirm the presence of diabetes.
Groupings: The experimental mice were given injections of streptozotocin to cause diabetes after two weeks of acclimation. Each week, the blood glucose level was measured with an accu-chek. For additional investigation, experimental animals judged to be diabetic (FBG>140 mg/dl) were used. Treatment started and continued in the following ways for 21 days:
| • | Group 1 (control): Group 1 vehicle treated: 10 mL/kg of DMSO solution (DMSO solution, 1 mL of DMSO and 19 mL of distill water i.e., 1:19) | |
| • | Group 2 (STZ only): Group 2 given a single dose of 55 mg/kg of STZ intraperitoneal (i.p) 8 | |
| • | Group 3 rats (STZ+METF): Group 3 given 55 mg/kg of STZ IP 8 and administration of 300 mg/kg of metformin25 | |
| • | Group 4 rats (STZ+MERC): Group 4 given 55 mg/kg of STZ IP 8 and 600 mg/kg MERC24 | |
| • | Group 5 rats (MERC only): Group 5 given 600 mg/kg of MERC24 |
Administration lasted for 21 days in all groups.
Animal sacrifice, blood/tissue collection and homogenization
Blood collection: Ketamine was used to anaesthetize the animals at a dose of 40 mg/kg of body weight26. Blood was drawn from the animals by cutting them open longitudinally from the abdominal region to the thoracic chamber, which houses the heart. Blood samples were collected into plain bottles and centrifuged for 15 min at 3000 ×g rpm to separate the serum. This was kept at -4°C until the assay of serum insulin concentration and antioxidant enzymes27-30.
Fasting blood glucose (FBG) level: The fasting blood glucose (FBG) in a blood sample drawn from the rats’ tail region was measured using an Acu-check glucometer and glucose strips31. For three weeks, the glucose levels of each rat were tested once a week.
Quantification of serum insulin: The radio-immunoassay approach was used to measure insulin32.
Procedure: On a microtiter plate, an antibody coating was coated with antiglobulin. The addition of antiglobulin antibody causes anti-insulin antibody to bind. Then, insulin (from sera, culture supernatants, or standard) is bound by an anti-insulin antibody. Whether insulin is enzyme-labeled or not, anti-insulin antibody competitive saturation occurs. The substrate degradation was evaluated after all unbound insulin, labelled or unlabeled, was extracted32.
Glycation activity measurement: According to Sega et al.33 states that the reaction mixture, which included 10 mg of bovine serum albumin (BSA) and 100 mg of d-glucose in 1 mL of sodium phosphate buffer (pH 7.2), was either incubated with or without the test material for two days at 60°C. The intensity of the fluorescence was measured using a fluorophotometer (Luminescence Spectrometer LS50B, Perkin-Elmer Ltd, Buckinghamshire, England) at 370 nm for excitation and 440 nm for emission. Addition of 2 mL of water to the reaction solution was made. The blank solution was the reaction mixture that contained no d-glucose. Aminoguanidine was used as the reference chemical in the duplicate assays34.
Oxidative stress studies: Superoxide dismutase (SOD) activity was assayed in line with the protocol described by Vilela et al35. Catalase (CAT) activity was determined by the protocol of Uchiyama and Mihara36.
Glutathione peroxidase activity: To determine GPX activity, glutathione oxidation is catalyzed by cumene hydroperoxide. Oxidized glutathione is reduced in the presence of NADPH and glutathione reductase, oxidizing NADPH to NADP+. Using hemolysates combined with reagent R1 and cumene R2, GPX activity is measured at 340 nm and expressed in U/mL in accordance with manufacturer instructions (Ransel Randox Lab, Antrim, UK). By thiols cleaving disulfide bonds to create TNB-, reduced glutathione (GSH) levels are determined using Ellman’s technique. Hemolysates are mixed with an assay buffer and Ellman reagents; TNB2- production is then measured at 412 nm after 15 min at room temperature. Findings are compared to a recognized GSH standard curve.
Malondialdehyde activity: The procedure for the thiobarbituric acid reaction was followed by Uchiyama and Mihara36. To measure the reactive components of thiobarbituric acid, absorption at 532 nm was compared to an MDA standard curve from the hydrolysis of 1,1,3,3-tetramethoxypropane under acid catalysis. A mixture of 0.25N hydrochloric acid, 15% trichloroacetic acid and 0.375% thiobarbituric acid was made. The 250 μL of serum and 500 μL of solution were added to each sample, which was then centrifuged at 3000×g rpm for 10 min after boiling for 10 min. To express MDA as μmol/L, 200 μL of each supernatant was applied to microplates and optical density was measured at 535 nm.
Statistical analysis: Mean±standard error of the mean was used to record the data. One-way ANOVA was used to examine the statistical difference between the means. The Tukey’s post-hoc test is employed to detect variations in individual mean values. GraphPad Prism 5, GraphPad Software, Inc., La Jolla, Calif., USA) recognized results with a value of p<0.05 as significant in all situations because the confidence interval was set at 95%.
RESULTS
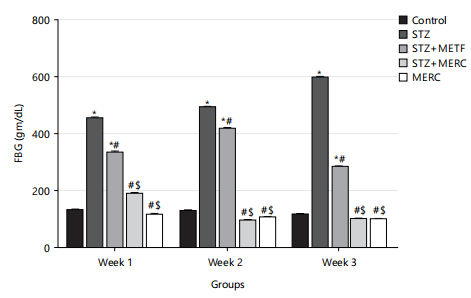
Impact of methanolic extract from Ricinus communis on fasting blood glucose levels in male Wistar rats rendered diabetics by streptozotocin: The fasting blood glucose (FBG) levels of the rats in the STZ and STZ+METF groups were considerably higher (p<0.05) than those in the control group. The FBG of the rats in the STZ+METF, STZ+MERC and MERC groups was significantly lower (p>0.05) than that of the STZ group. The rats in the STZ+MERC and MERC groups had significantly (p<0.05) lower blood glucose levels than the FBG of the STZ+METF group. The FBG of rats in the MERC group was not significantly lower (p>0.05) than that of rats in the STZ+MERC group as shown in Fig 1.
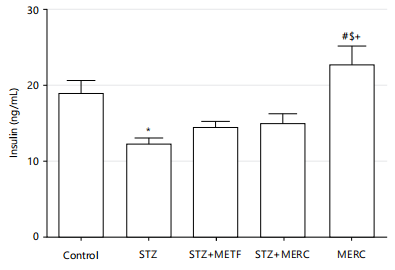
Impact of methanolic extract from Ricinus communis on insulin concentration in male Wistar rats rendered diabetics by streptozotocin: When compared to the insulin level of the control group, the rats in the STZ, STZ+METF and STZ+MERC groups had insignificantly lower insulin levels (p>0.05) and the rats in the MERC group had insignificantly higher insulin levels (p>0.05). When compared to the insulin level of rats in the STZ group, the insulin levels of rats in the STZ+METF and STZ+MERC groups were not statistically different (p>0.05). When compared to the insulin level of the rats in the STZ group, the rats in the MERC group had a considerably higher insulin level (p<0.05). When compared to the insulin level in the STZ+METF group, the rats in the MERC group had an insignificantly higher insulin level (p>0.05), but the rats in the STZ+MERC group had a significantly higher insulin level (p<0.05) as shown in Fig. 2.
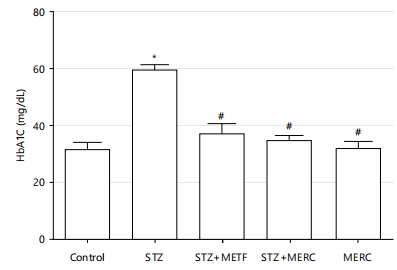
Impact of methanolic extract from Ricinus communis on glycated haemoglobin in male Wistar rats rendered diabetics by streptozotocin: Rats in the STZ group had significantly higher levels of glycated haemoglobin (HbA1C) (p<0.05) than rats in the control group. Rats in the STZ+METF, STZ+MERC and MERC groups had slightly higher HbA1C levels (p>0.05) than the control group. Rats in the STZ+METF, STZ+MERC and MERC groups had significantly lower HbA1C levels (p<0.05) than the rats in the STZ group. The HbA1C levels of the rats in the STZ+METF, STZ+MERC and MERC groups do not differ significantly (p>0.05) as described in Fig. 3.
|
|
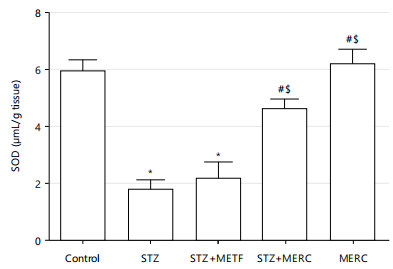
Impact of methanolic extract from Ricinus communis on oxidative stress marker (SOD and CAT) in male Wistar rats rendered diabetics by streptozotocin: In comparison to the control group, the SOD activity of the rats in the STZ and STZ+METF groups considerably decreased (p<0.05), but it rose insignificantly (p>0.05) in the STZ+MERC group. The SOD activity of the MERC group and the control group did not differ significantly. In contrast to the STZ group, SOD activity rose significantly (p<0.05) in the STZ+MERC and MERC groups. Furthermore, compared to the STZ+METF group, SOD activity rose significantly (p<0.05) in the STZ+MERC and MERC groups. Comparing the MERC group to the STZ+MERC group, there was no discernible increase in SOD activity as shown in Fig. 4.
|
|
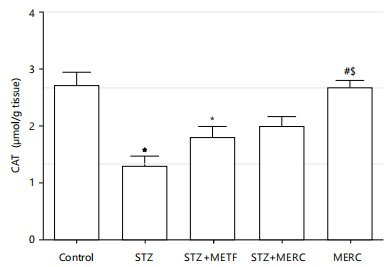
When compared to the control group, the catalase (CAT) activity in the STZ and STZ+METF groups dramatically dropped (p<0.05). When compared to the control group, the STZ+MERC group’s drop in CAT activity was not statistically significant. The MERC group’s and the control group’s CAT activity did not differ significantly. When compared to the STZ group, the MERC group had a significant increase (p<0.05) in CAT activity, but the STZ+METF and STZ+MERC groups showed minor increases (p>0.05). When compared to the STZ+METF group, CAT activity increased considerably in the MERC group but insignificantly (p>0.05) not the STZ+MERC group. In comparison to the STZ+MERC group, the CAT activity in the MERC group increased insignificantly (p>0.05) (Fig. 5).
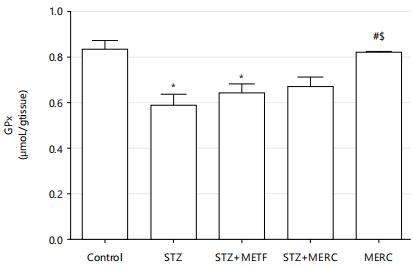
Impact of methanolic extract from Ricinus communis on oxidative stress marker (GPx and MDA) in male Wistar rats rendered diabetics by streptozotocin: Rats from the STZ and STZ+METF groups had significantly lower GPx levels (p<0.05) than the control group. Comparing the STZ+MERC and MERC groups to the control group, however, revealed negligible decreases in GPx levels (p>0.05). The GPx levels in the STZ+METF and STZ+MERC groups increased marginally (p>0.05) in comparison to the STZ group. In contrast to the STZ group, the GPx levels in the MERC group increased significantly (p<0.05). In comparison to the STZ+METF group, the rise in GPx levels in the STZ+MERC group was negligible. When compared to the STZ+METF group, the GPx levels in the MERC group increased significantly (p<0.05), but not significantly (p>0.05) in comparison to the STZ+MERC group (Fig. 6).
|
|
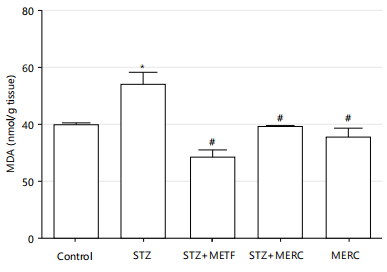
When compared to the control group, the MDA levels in the rats from the STZ group increased considerably (p<0.05). When compared to the control group, the MDA levels in the STZ+METF, STZ+MERC and MERC groups reduced marginally. The MDA levels in the STZ+METF, STZ+MERC and MERC groups considerably decreased (p<0.05) in comparison to the STZ group. When compared to the STZ+METF group, the MDA levels in the STZ+MERC and MERC groups exhibited minor increases (p>0.05), while the MERC group showed an insignificant decrease (p>0.05) in comparison to the STZ+MERC group as shown in Fig 7.
|
DISCUSSION
Diabetes is a serious health problem all over the world. It causes high levels of sugar in the blood and can lead to illness and death. Although glucose is essential for cellular energy, excessive concentrations can lead to various complications6. Therefore, managing blood glucose levels is crucial to mitigate associated risks. Administration of methanolic extract of Ricinus communis leaves to STZ-induced diabetic rats resulted in reduced blood glucose levels, consistent with findings by Gad-Elkareem et al.37. Insulin, produced by pancreatic beta cells, plays a pivotal role in metabolic regulation, facilitating glucose absorption into liver, fat and muscle cells38. Reduced insulin levels in STZ-induced rats were restored in the STZ+MERC rats, indicating MERC’s insulinemic effect. Alkaloids, flavonoids and saponins present in MERC may stimulate insulin release or inhibit glucose absorption in the intestines39.
Glycation occurs when glucose and fructose react with proteins and lipids, forming advanced glycation end products (AGEs). This process leads to the production of ketoamines when proteins and reducing sugars condense40. Any protein with reactive sites can undergo glycation and the level of glycated proteins in the bloodstream serves as a marker for long-term changes in blood glucose levels. In the rats treated with STZ, glycation activity increased. Elevated AGE levels, as observed in diabetic nephropathy (DN) cases, may exacerbate symptoms due to increased blood glucose levels, which accelerate degenerative changes in the kidneys by activating the mitogen-activated protein kinase/extracellular regulated protein kinase (MAPK/ERK) pathway41. Compounds that interfere with the glycation pathway have demonstrated protective effects on organs affected by diabetic complications42.
The STZ administration disrupts renal antioxidant activity, leading to decreased levels of SOD, CAT and GPx, increased malondialdehyde production and lipid peroxidation43. Ricinus communis leaf extract exhibits significant antioxidant activity in renal tissue, likely attributed to flavonoids and alkaloids content inhibiting oxidative stress pathways44. The SODs are crucial antioxidants that defend against reactive oxygen species (ROS), catalyzing the dismutation of superoxide radicals into less harmful molecules45. Catalase (CAT) and GPx further neutralize ROS, reducing oxidative damage in the kidney as described by Wang et al.43. Reduced levels of malondialdehyde (MDA) in the STZ+MERC group indicate the extract’s ability to mitigate lipid peroxidation and oxidative stress44.
In streptozotocin-induced diabetic male Wistar rats, the study on Ricinus communis L. extracts demonstrates strong hypoglycemic, insulin regulation and antioxidant effects, suggesting possible therapeutic uses for diabetes treatment. Applications include the creation of natural antioxidant supplements and novel diabetes therapies. More research is advised to ascertain the safe dosage, long-term impacts and human mechanisms of action. The study’s limitations include its dependence on animal models, which could not accurately represent human physiology and the requirement for human clinical trials to verify efficacy and safety. In addition, Ricinus communis’s possible toxicity needs to be carefully considered.
CONCLUSION
The present study demonstrated that the methanolic extract of Ricinus communis L. (castor bean) has significant therapeutic effects in the treatment of streptozotocin-induced diabetes in male Wistar rats. The results showed that castor extract effectively reduced blood glucose levels and improved antioxidant status in diabetic rats. These results were comparable to and in some respects exceeded those of metformin, a standard antidiabetic drug. The results suggest that castor extract has the potential to serve as a natural alternative or complementary treatment for diabetes due to its diverse and relative benefits and low side effect profile. However, further research, including human clinical trials, is needed to fully elucidate the mechanisms involved and confirm its effectiveness and safety in a broader population. Overall, this study highlights the therapeutic potential of Ricinus communis L. in diabetes treatment and opens opportunities for the development of novel plant-based antidiabetic therapies.
SIGNIFICANCE STATEMENT
The potential for this effort to result in the development of novel plant-based diabetes therapies makes it significant. The effectiveness of an extract from Ricinus communis L., which has been used in traditional medicine, is assessed in a diabetic rat model to see if it can lower oxidative stress and improve insulin and blood glucose levels. Its potential as a substitute or adjunctive treatment can be better understood by contrasting its effects with those of metformin, a common antidiabetic medication. This study may result in the creation of cutting-edge, all-natural medications with fewer adverse effects, providing a more comprehensive approach to the management of diabetes and enhancing patient outcomes.
REFERENCES
- Olokoba, A.B., O.A. Obateru and L.B. Olokoba, 2012. Type 2 diabetes mellitus: A review of current trends. Oman Med. J., 27: 269-273.
- Ale, A.O. and O. Odusan, 2019. Spectrum of endocrine disorders as seen in a tertiary health facility in Sagamu, Southwest Nigeria. Niger. Med. J., 60: 252-256.
- Zhao, M., M. Xiao, Q. Tan and F. Lu, 2023. Triglyceride glucose index as a predictor of mortality in middle-aged and elderly patients with type 2 diabetes in the US. Sci. Rep., 13.
- Adeloye, D., J.O. Ige, A.V. Aderemi, N. Adeleye, E.O. Amoo, A. Auta and G. Oni, 2017. Estimating the prevalence, hospitalisation and mortality from type 2 diabetes mellitus in Nigeria: A systematic review and meta-analysis. BMJ Open, 7.
- Hill, M.A., Y. Yang, L. Zhang, Z. Sun, G. Jia, A.R. Parrish and J.R. Sowers, 2021. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism, 119.
- Suh, J., Y. Choi, J.S. Oh, K. Song and H.S. Choi et al., 2023. Association between early glycemic management and diabetes complications in type 1 diabetes mellitus: A retrospective cohort study. Primary Care Diabetes, 17: 60-67.
- Pieper, A.A., D.J. Brat, D.K. Krug, C.C. Watkins and A. Gupta et al., 1999. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA, 96: 3059-3064.
- Ghasemi, A. and S. Jeddi, 2023. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. EXCLI J., 22: 274-294.
- Frost, P.A., S. Chen, M.J. Mezzles, V.S. Voruganti and E.J. Nava-Gonzalez et al., 2015. Successful pharmaceutical-grade streptozotocin (STZ)-induced hyperglycemia in a conscious tethered baboon (Papio hamadryas) model. J. Med. Primatol., 44: 202-217.
- Siddiqui, M., A. Vora, S. Ali, J. Abramowitz and S. Mirfakhraee, 2021. Pasireotide: A novel treatment for tumor-induced hypoglycemia due to insulinoma and non-islet cell tumor hypoglycemia. J. Endocr. Soc., 5.
- Akinlade, O.M., B.V. Owoyele and A.O. Soladoye, 2021. Streptozotocin-induced type 1 and 2 diabetes in rodents: A model for studying diabetic cardiac autonomic neuropathy. Afr. Health Sci., 21: 719-727.
- Qasim, N., A. Arif and R. Mahmood, 2023. Hyperglycemia enhances the generation of ROS and RNS that impair antioxidant power and cause oxidative damage in human erythrocytes. Biochem. Cell Biol., 101: 64-76.
- Ayala, A., M.F. Muñoz and S. Argüelles, 2014. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longevity, 2014.
- Pan, Q., X. Lu, C. Zhao, S. Liao and X. Chen et al., 2020. Metformin: The updated protective property in kidney disease. Aging, 12: 8742-8759.
- Mahmood, R., D. Maccourtney, M. Vashi and A. Mohamed, 2023. A case of metformin-associated lactic acidosis. Cureus, 15.
- Ekor, M., 2014. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol., 4.
- Elkordy, A.A., R.R. Haj-Ahmad, A.S. Awaad and R.M. Zaki, 2021. An overview on natural product drug formulations from conventional medicines to nanomedicines: Past, present and future. J. Drug Delivery Sci. Technol., 63.
- Chouhan, H.S., G. Swarnakar and B. Jogpal, 2021. Medicinal properties of Ricinus communis: A review. Int. J. Pharm. Sci. Res., 12: 3632-3642.
- Singh, R., A. Barden, T. Mori and L. Beilin, 2001. Advanced glycation end-products: A review. Diabetologica, 44: 129-146.
- Altemimi, A., N. Lakhssassi, A. Baharlouei, D.G. Watson and D.A. Lightfoot, 2017. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants, 6.
- Marwat, S.K., Fazal-Ur-Rehman, E.A. Khan, M.S. Baloch and M. Sadiq et al., 2017. Review-Ricinus cmmunis: Ethnomedicinal uses and pharmacological activities. Pak. J. Pharm. Sci., 30: 1815-1827.
- Abdul, W.M., N.H. Hajrah, J.S.M. Sabir, S.M. Al-Garni and M.J. Sabir et al., 2018. Therapeutic role of Ricinus communis L. and its bioactive compounds in disease prevention and treatment. Asian Pac. J. Trop. Med., 11: 177-185.
- Verheijen, M., M. Lienhard, Y. Schrooders, O. Clayton and R. Nudischer et al., 2019. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep., 9.
- Adeoye, S.W.A., O.A. Adeshina, M.G. Yusuf and A.I. Omole, 2022. Hepatoprotective and renoprotective effect of Moringa oleifera seed oil on Dichlorvos-induced toxicity in male Wistar rats. Niger. J. Physiol. Sci., 37: 119-126.
- Murai, Y., T. Ohta, H. Tadaki, K. Miyajima, M. Shinohara, F. Fatchiyah and T. Yamada, 2017. Assessment of pharmacological responses to an anti-diabetic drug in a new obese type 2 diabetic rat model. Med. Arch., 71: 380-384.
- Igbayilola, Y.D. and M.G. Gujja, 2024. Alpha-amylase and alpha-glucosidase upregulated glucose homeostasis in high-fat fed Wistar rats supplemented with cocoa flavonoid-rich aqueous. Food Biosci., 59.
- Igbayilola, Y.D., A.O. Morakinyo and B.O. Iranloye, 2021. Adverse effects of perinatal protein restriction on glucose homeostasis in offspring of Sprague-Dawley rats. Sci. Afr., 14.
- Dimeji, I.Y., A.O. Samson, A. Kayode, A. Ikponwosa, W.O. Dada and O.F. Adesina, 2023. Glucometabolic response to walnut (Juglans regia L.) supplementation during gestation and/or lactation in offspring of Sprague-Dawley rats. Singapore J. Sci. Res., 13: 79-87.
- Igbayilola, Y., A. Morakinyo and B. Iranloye, 2021. Leptin-resistance induced hyperphagia and diminished oxidative balance in offspring of dams exposed to perinatal protein restriction. Afr. J. Biomed. Res., 24: 451-458.
- Dimeji, I.Y., M.A. Olufemi and O.I. Bolanle, 2021. Adverse effects of perinatal protein restriction on regulators of lipid metabolism and hepatic function in offspring of Sprague-Dawley rats. Niger. J. Exp. Clin. Biosci., 9: 74-81.
- Kennard, M.R., L.F.D. Gatward, A.G. Roberts, E.R.P. White, M. Nandi and A.J.F. King, 2021. The use of mice in diabetes research: The impact of experimental protocols. Diabetic Med., 38.
- Warnken, T., K. Huber and K. Feige, 2016. Comparison of three different methods for the quantification of equine insulin. BMC Vet. Res., 12.
- Sega, M.F., H. Chu, J.A. Christian and P.S. Low, 2015. Fluorescence assay of the interaction between hemoglobin and the cytoplasmic domain of erythrocyte membrane band 3. Blood Cells Mol. Dis., 55: 266-271.
- Cho, S., V.A. Duong, J.H. Mok, M. Joo, J.M. Park and H. Lee, 2022. Enrichment and analysis of glycated proteins. Rev. Anal. Chem., 41: 83-97.
- Vilela, D.D., L.G. Peixoto, R.R. Teixeira, N.B. Baptista and D.C. Caixeta et al., 2016. The role of metformin in controlling oxidative stress in muscle of diabetic rats. Oxid. Med. Cell. Longevity, 2016.
- Uchiyama, M. and M. Mihara, 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem., 86: 271-278.
- Gad-Elkareem, M.A.M., E.H. Abdelgadir, O.M. Badawy and A. Kadri, 2019. Potential antidiabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ, 7.
- Zhao, L., L. Wang, Y. Zhang, S. Xiao and F. Bi et al., 2017. Glucose oxidase-based glucose-sensitive drug delivery for diabetes treatment. Polymers, 9.
- Kibiti, C.M. and A.J. Afolayan, 2015. Herbal therapy: A review of emerging pharmacological tools in the management of diabetes mellitus in Africa. Pharmacogn. Mag., 11: S258-S274.
- Sourris, K.C., A. Watson and K. Jandeleit-Dahm, 2020. Inhibitors of Advanced Glycation End Product (AGE) Formation and Accumulation. In: Reactive Oxygen Species: Network Pharmacology and Therapeutic Applications, Schmidt, H.H.H.W., P. Ghezzi and A. Cuadrado (Eds.), Springer, Cham, Switzerland, ISBN: 978-3-030-68510-2, pp: 395-423.
- Genuth, S., W. Sun, P. Cleary, D.R. Sell and W. Dahms et al., 2005. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes, 54: 3103-3111.
- Mengstie, M.A., E.C. Abebe, A.B. Teklemariam, A.T. Mulu and M.M. Agidew et al., 2022. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front. Mol. Biosci., 9.
- Wang, F., H. Sun, B. Zuo, K. Shi, X. Zhang, C. Zhang and D. Sun, 2021. Metformin attenuates renal tubulointerstitial fibrosis via upgrading autophagy in the early stage of diabetic nephropathy. Sci. Rep., 11.
- Gwarzo, M.Y., V.A. Nwachuku and A.O. Lateef, 2010. Prevention of alloxan induced diabetes mellitus in rats by vitamin a dietary supplementation. Asian J. Anim. Sci., 4: 190-196.
- Younus, H., 2018. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci., 12: 88-93
How to Cite this paper?
APA-7 Style
Dimeji,
I.Y., Adeoye,
S.W., Ifeoluwa,
A.V., Ezekiel,
A.A. (2024). Hypoglycaemic, Insulin Modulation and Antioxidant Potentials of Ricinus communis L. Extracts in Streptozotocin-Induced Diabetes Male Wistar Rats. Research Journal of Medicinal Plants, 18(1), 41-54. https://doi.org/10.3923/rjmp.2024.41.54
ACS Style
Dimeji,
I.Y.; Adeoye,
S.W.; Ifeoluwa,
A.V.; Ezekiel,
A.A. Hypoglycaemic, Insulin Modulation and Antioxidant Potentials of Ricinus communis L. Extracts in Streptozotocin-Induced Diabetes Male Wistar Rats. Res. J. Med. Plants 2024, 18, 41-54. https://doi.org/10.3923/rjmp.2024.41.54
AMA Style
Dimeji
IY, Adeoye
SW, Ifeoluwa
AV, Ezekiel
AA. Hypoglycaemic, Insulin Modulation and Antioxidant Potentials of Ricinus communis L. Extracts in Streptozotocin-Induced Diabetes Male Wistar Rats. Research Journal of Medicinal Plants. 2024; 18(1): 41-54. https://doi.org/10.3923/rjmp.2024.41.54
Chicago/Turabian Style
Dimeji, Igbayilola,, Yusuff, Saka, Waidi Adeoye, Adisa, Victoria Ifeoluwa, and Adeogun Adetomiwa Ezekiel.
2024. "Hypoglycaemic, Insulin Modulation and Antioxidant Potentials of Ricinus communis L. Extracts in Streptozotocin-Induced Diabetes Male Wistar Rats" Research Journal of Medicinal Plants 18, no. 1: 41-54. https://doi.org/10.3923/rjmp.2024.41.54

This work is licensed under a Creative Commons Attribution 4.0 International License.



